Explore a decade of impactful milestones in the fight against Limb-Girdle Muscular Dystrophy (LGMD). From groundbreaking genetic sequencing and awareness campaigns to international conferences and clinical trials, discover the significant advancements and ongoing efforts to improve the lives of those affected by LGMD.
2014
In 2014, free next-generation genetic sequencing for LGMDs (35 genes) was made available to US patients through an LGMD advocacy program, resulting in thousands of free tests and genetic diagnoses.
LGMD-Info.org, the “Information Hub for the LGMD community” was created.

The Lime Green for LGMD awareness campaign was introduced and continues to be our signature color.

The Clinical Outcome Study for Dysferlinopathy (COS) was conducted, marking a significant international effort to understand the disease.
2015

The first LGMD Awareness Day was celebrated on September 30, 2015.
The first state proclamations were received, declaring September 30th as Limb-Girdle Muscular Dystrophy Awareness Day in Wisconsin, Washington, Missouri, and Colorado.

The Dysferlin Scientific Conference was held in Toronto, Canada, gathering researchers, clinicians, and industry experts to share insights and advancements in the study and treatment of dysferlinopathy, a form of Limb-Girdle Muscular Dystrophy.
The 2B Empowered Patient Conference was held in Dallas, Texas, providing a platform for patients with Limb-Girdle Muscular Dystrophy to connect, share experiences, and gain valuable insights from experts in the field.
2016

In 2016, 219th ENMC International Workshop on Titinopathies: An international database of titin mutations and phenotypes was held in Heemskerk, The Netherlands.

The Samantha J. Brazzo Foundation rebranded to CureLGMD2i. This change reflected the foundation’s dedicated focus on finding a cure for Limb-Girdle Muscular Dystrophy Type 2i (LGMD2i).
CureLGMD2i and the LGMD2i Research Fund supported the first-ever clinical trial in LGMD2I/R9, titled “A Trial of PF-06252616 in Ambulatory Participants with LGMD2I,” led by Dr. Kathryn Wagner.

Dysferlin Registry launched a new platform specifically for patients with genetically confirmed dysferlinopathy.
Dual vector gene therapy for dysferlinopathy, specifically targeting Limb-Girdle Muscular Dystrophy Type, received clearance from the FDA for its Investigational New Drug (IND) application.
The second 2B Empowered Patient Conference took place in Dallas, Texas.
2017

Myonexus Therapeutics was founded in 2017 to develop gene therapies for treating five subtypes of Limb-Girdle Muscular Dystrophy (LGMD): LGMD2B/R2, LGMD2C/R5, LGMD2D/R5, LGMD2E/R4, and LGMD2L/R12. This clinical-stage biotech company, in collaboration with Nationwide Children’s Hospital, focuses on creating first-ever corrective treatments for these forms of LGMD, utilizing an innovative gene therapy platform based on the AAVrh.74 vector system (Sarepta Therapeutics) (Muscular Dystrophy News) (BioSpace).
The 229th ENMC international workshop in Naarden, Netherlands, March 17–19, 2017, developed a new nomenclature and classification system for limb girdle muscular dystrophies (LGMDs). The revision was necessary to update the definition of LGMD and address the issue of the recessive form’s subtype numbering reaching Z (LGMD 2Z). The new system aims to make LGMD a more distinct entity with commonalities among subtypes.
In 2017, the first intra-muscular (IM) dual vector gene therapy clinical trial for dysferlinopathy was initiated, marking a significant advancement in the treatment of this form of LGMD.
2018

In 2018, the Genetic Resolution and Assessments Solving Phenotypes in LGMD (GRASP-LGMD) consortium was created to hasten therapeutic development in the LGMDs.

The LGMD2L Foundation was established.

The Coalition to Cure Calpain 3 announced their Gene Therapy Initiative, aimed at accelerating the development of gene therapies to treat Limb-Girdle Muscular Dystrophy type 2A/R1 (LGMD2A/R1).

The LGMD2A/R1 Scientific Conference was held in Arlington, Virginia, bringing together experts and researchers to discuss the latest advancements and strategies in the fight against Limb-Girdle Muscular Dystrophy type 2A/R1.

The LGMD-1D DNAJB6 Foundation was established.
Sarepta Therapeutics conducted the first whole-body administration of gene therapy for Limb-Girdle Muscular Dystrophy, specifically targeting the beta-Sarcoglycan gene (LGMD2E).
2019
In 2019, the International LGMD Community Advisory Board (CAB) was established to provide a unified voice for the global Limb-Girdle Muscular Dystrophy community and to facilitate collaboration between patients, researchers, and healthcare providers.

The “LIMEmoji Challenge” to help raise awareness of LGMD was introduced.
A clinical study was initiated to evaluate the open label safety and efficacy of a once-weekly steroid treatment in patients with Limb-Girdle Muscular Dystrophy (LGMD).

The first International LGMD Conference was held in Chicago, Illinois, bringing together patients, families, researchers, and clinicians from around the world to share knowledge and advance the understanding and treatment of Limb-Girdle Muscular Dystrophy (LGMD).

The 2B Empowered patient meeting took place in Mumbai, India, providing a platform for individuals affected by Limb-Girdle Muscular Dystrophy to connect, share experiences, and gain valuable insights from leading experts in the field.
2020

In 2020, the LGMD Awareness Foundation was established to raise awareness, provide education, and support research efforts aimed at improving the lives of those affected by Limb-Girdle Muscular Dystrophy (LGMD).

Girdie, the LGMD Ambassador, was introduced to the LGMD community.
A Patient Listening Session for Limb-Girdle Muscular Dystrophy (LGMD) was held with the FDA, providing patients and caregivers an opportunity to share their experiences and highlight the urgent need for new treatments and therapies directly with regulatory officials.
2021

The first issue of LGMD News magazine was published (vol 1/issue 1) in 2021.
The first “Virtual” International LGMD Conference was held, allowing participants from around the world to connect and engage in discussions about the latest research, treatments, and support for Limb-Girdle Muscular Dystrophy (LGMD) despite the challenges posed by the global pandemic.
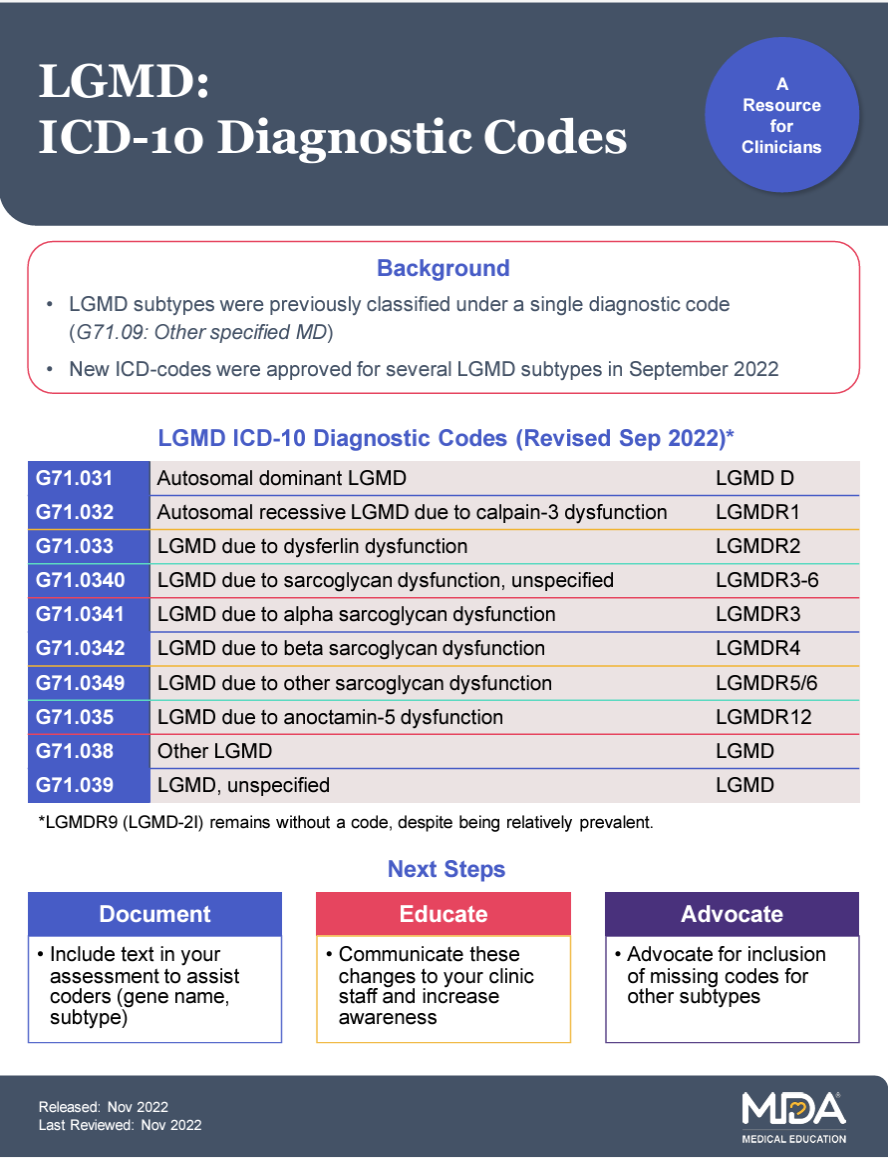
Dedicated ICD-10 medical codes were approved for several specific subtypes of Limb-Girdle Muscular Dystrophy (LGMD) as well as for non-specified subtypes, facilitating better diagnosis, treatment, and tracking of the disease in the healthcare system.
The first patient was dosed in a Phase 2 clinical trial of BBP-418 (Ribitol) for the treatment of Limb-Girdle Muscular Dystrophy Type 2I/R9 (LGMD2I/R9), conducted by BridgeBio Pharma and its affiliate ML Bio Solutions.
2022

In 2022, the LGMD Global Advocacy Summit convened, bringing together advocates, patients, researchers, and policymakers from around the world to collaborate and advance efforts in raising awareness, supporting research, and improving the lives of those affected by Limb-Girdle Muscular Dystrophy (LGMD).

Girdie, the LGMD ambassador, was “brought to life” and debuted at the Wellstone Dystroglycanopathies Patient & Family Conference in Iowa.

The first Externally Led Patient-Focused Drug Development Meeting (EL-PFDD) for Limb-Girdle Muscular Dystrophies (LGMDs) was held with the FDA and other stakeholders, providing a crucial platform for patients and caregivers to share their experiences and perspectives to inform drug development and regulatory decision-making.
The CDC instituted LGMD-specific ICD-10 codes, enabling more precise diagnosis, documentation, and tracking of Limb-Girdle Muscular Dystrophy subtypes within the healthcare system.

The nonprofit Team Titin, Inc. was formed, which supports the LGMD2J/R10 community.
Team Titin hosted the SciFam Research-Family Conference.

The Daniel Ferguson LGMD Foundation was established in Australia, dedicated to supporting research, raising awareness, and providing resources for individuals and families affected by Limb-Girdle Muscular Dystrophy.
The first patient was dosed in a Phase 1/2 clinical trial in Europe of gene therapy for LGMD-R9, conducted by Atamyo Therapeutics, marking a significant step forward in the development of treatments for this subtype of Limb-Girdle Muscular Dystrophy.

CureLGMD2i hosted the first “Connecting for a Cure” event on LGMD Awareness Day, bringing together patients, families, researchers, and advocates to raise awareness, share knowledge, and support the search for a cure for Limb-Girdle Muscular Dystrophy type 2i.
The Clinical Outcome Study/Natural History of Dysferlinoapthy celebrated 10 years of patient participation and clinical data collection, highlighting a decade of valuable contributions to the understanding and treatment of this specific form of Limb-Girdle Muscular Dystrophy.

LGMD Advocacy Bundles were deployed at 6 clinics in the US by LGMD Awareness Foundation.
2023

In 2023, the LGMD “Voice of the Patient” Report was published, highlighting the unmet needs and challenges faced by individuals with six specific subtypes of Limb-Girdle Muscular Dystrophy, and providing valuable insights to guide future research and treatment efforts.
A study was initiated to evaluate the efficacy and safety of BBP-418 (Ribitol) in patients with Limb-Girdle Muscular Dystrophy 2I (LGMD2I/R9), conducted by ML BioSolutions.
The first patient was treated in a Phase 1/Phase 2 trial of AB-1003 gene therapy for Limb-Girdle Muscular Dystrophy Type 2I/R9, conducted by AskBio, marking a significant milestone in the development of new therapies for this LGMD subtype.

The first “hybrid” International LGMD Conference was held in Washington, DC, combining both in-person and virtual participation to accommodate a global audience and foster collaboration among researchers, clinicians, patients, and advocates in the fight against Limb-Girdle Muscular Dystrophy.

Coalition to Cure Calpain 3 launches new LGMD2A/Calpainopathy Registry

The nonprofit The Dion Foundation was established, dedicated to supporting research, raising awareness, and providing resources for individuals and families affected by Limb-Girdle Muscular Dystrophy.
Sarepta Therapeutics initiated the first whole body/systemic treatment in the SRP 6004 Proof of Concept Clinical Trial, part of the NAVIGENE study of dysferlinopathy, representing a significant advancement in the treatment approach for this specific form of Limb-Girdle Muscular Dystrophy.
2024

In 2024, screening was initiated in the EMERGENE Phase 3 Clinical Study of SRP-9003 for the treatment of Limb-Girdle Muscular Dystrophy Type 2E/R4, conducted by Sarepta Therapeutics, marking a crucial step towards potential new treatment options for this LGMD subtype.

The LGMD Scientific Drug Development Workshop was held in Bethesda, Maryland, bringing together researchers, clinicians, and industry leaders to discuss and advance the development of new therapeutic strategies for Limb-Girdle Muscular Dystrophy.

The clinical trial application for testing ATA-200, an investigational gene therapy for children with Limb-Girdle Muscular Dystrophy type 2C/R5, was approved in France and Italy by Atamyo Therapeutics, paving the way for crucial research into potential treatments for this LGMD subtype.

The first LGMDR9 (LGMD2I) European Patient Day was held in Amsterdam, The Netherlands, providing a platform for patients, families, researchers, and healthcare professionals to connect, share experiences, and learn about the latest advancements in the treatment and management of Limb-Girdle Muscular Dystrophy Type 2I.
The LGMD2A/R1 Scientific Meeting was held in Arlington, Virginia, bringing together experts and researchers to discuss the latest findings and advancements in the understanding and treatment of Limb-Girdle Muscular Dystrophy Type 2A/R1.
The Dysferlin Scientific Conference was held in Houston, Texas, gathering researchers, clinicians, and industry experts to share insights and advancements in the study and treatment of dysferlinopathy, a form of Limb-Girdle Muscular Dystrophy.

The 10th Annual LGMD Awareness Day is celebrated worldwide.